Günter Seyfried
Clemens Grabher
Daniel Feurle
2008
In this experiment, we encoded a film sequence into a string of GATC with our software. Based on that new information a DNA strand was synthesized by DNA 2.0. In vitro, we exposed the encoded film sequences to conditions of simulated radioactivity, UV radiation and toxicity. As a result, we recovered the mutated information and re-translated it into a film sequence. In the artistic investigation, interesting questions regarding the circumstances of how a piece of code could transcend itself into a piece of art and a subsequent set of problems regarding a possible self-generation of art fragments, came to light. We also found out, that the involvement of “visual material” during the biological processes (situated as a corporeal part of the process), establishes a presence of the eye and therefore the mind.
In a first step we translated a small film (film_2_1.gif – 8 x 8 pixel, 2 frames, 4 colours) with our software DNASplitter into a string of GATC.
film_2_1.gif ![]()



Frame 1 (zoomed) Frame 2 (zoomed)
High-Fidelity PCR cited from the website of promega
For some applications, such as gene expression, mutagenesis or cloning, the number of mutations introduced during PCR needs to be minimized. For these applications, we recommend using a proofreading polymerase. Proofreading DNA polymerases, such as Pfu and Tli DNA polymerases, have a 3′→5′ exonuclease activity, which can remove any misincorporated nucleotides, and so the error rate is relatively low. The accuracy of Pfu DNA polymerase is approximately twofold higher than that of Tli DNA polymerase and 6-fold higher than that of Taq DNA polymerase (Cline, 1996).
The most commonly used DNA polymerase for PCR is Taq DNA polymerase, which has an error rate of approximately 1 × 10–5 errors per base. This error rate is relatively high due to the enzyme’s lack of 3′→5′ exonuclease (proofreading) activity. The error rate of Tfl DNA polymerase, another nonproofreading polymerase, is similar to that of Taq DNA polymerase.
Reaction conditions can affect DNA polymerase fidelity, and DNA polymerases may be affected in different ways or to different degrees. In general, excess magnesium or the presence of manganese will cause the fidelity of DNA polymerases to be reduced (Eckert and Kunkel, 1990). Unequal nucleotide concentrations can also affect fidelity; nucleotides that are present at higher concentrations will be misincorporated at a higher frequency (Eckert and Kunkel, 1990). Reaction pH can also have a big effect on fidelity (Eckert and Kunkel, 1990; Eckert and Kunkel, 1991). For example, the fidelity of Taq DNA polymerase increases as pH decreases, with the lowest error rate occurring in the range of pH 5–6 (Eckert and Kunkel, 1990), but the opposite is true for Pfu DNA polymerase. Pfu DNA polymerase has higher fidelity at higher pH (Cline, 1996). Finally, exposing the DNA template to very high temperatures (i.e., 94°C) for extended periods of time can lead to DNA damage, specifically the release of bases from the phosphodiester backbone. The resulting abasic sites can cause some DNA polymerases to stall but can also result in a higher rate of mutations, most frequently transversions, as the DNA polymerase adds a random nucleotide at an abasic site (Eckert and Kunkel, 1991).
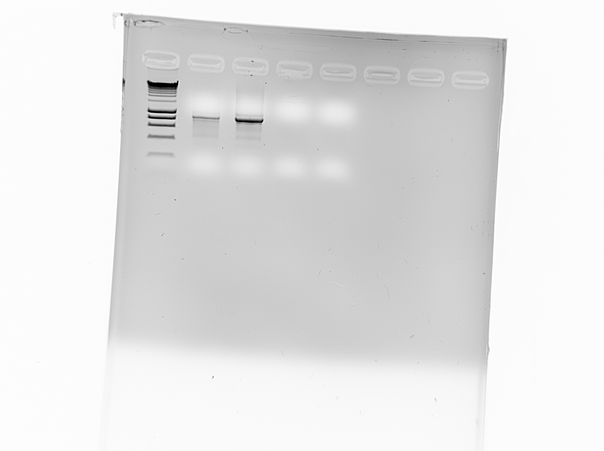
The synthesized DNA sequence, the artificial gene, served as a template for PCR reactions under the conditions mentioned above, which resulted in different error rates during the replication of the artificial gene. After the PCR reaction took place under “sup-optimal conditions” the products were inserted into cloning vectors to facilitate the sequencing later on.
We obtained the sequences, that deviated from the initial template.
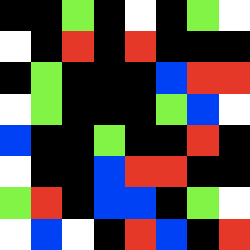
mu1_22.gif ![]()
strong mutations in both frames at a higher MgCl- concentration
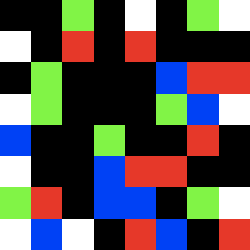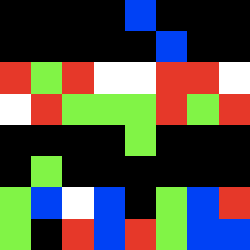
Frame 1 (zoomed) Frame 2 (zoomed)
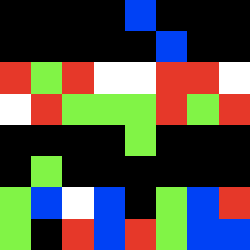
mu2_24.gif ![]()
moderate mutations in the first frame using the same parameter



Frame 1 (zoomed) Frame 2 (zoomed)
The results visualized in an interactive installation:

